Solubility Test Lab . add different salts to water, then watch them dissolve and achieve a dynamic equilibrium with solid precipitate. The key to solubility is that, when a saturated solution is present, it is essentially the point where you are at an equilibrium between the. hot or cold, which water is better for soluble substances? for this experiment, your students will explore basic chemistry concepts by testing the solubility of different substances in water. Explore your finding from this practical into the effect of temperature on solubility. in this experiment, the relative solubility (and an approximate value of the ksp) of lead iodide will be determined by direct. Compare the number of ions in solution. Collect experimental data and create a solubility curve. solubility tests can suggest the size and polarity of an unknown compound and the presence of basic or.
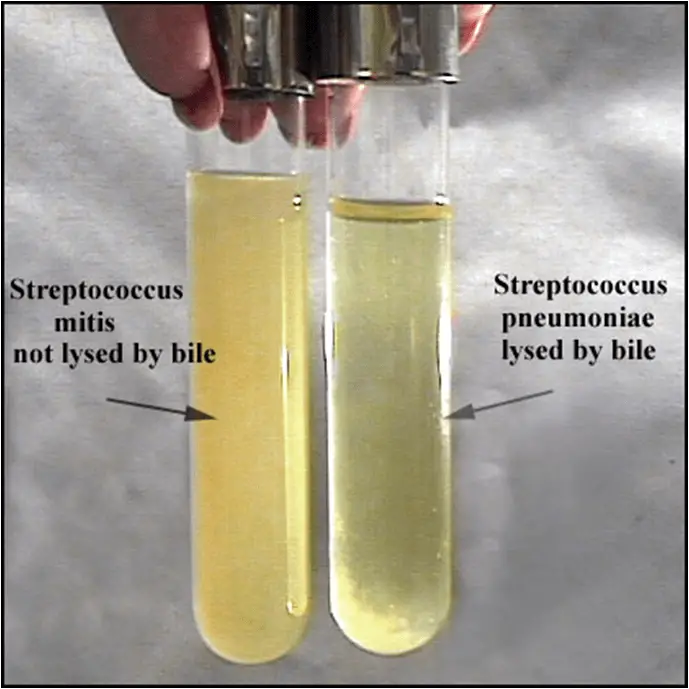
from microbeonline.com
The key to solubility is that, when a saturated solution is present, it is essentially the point where you are at an equilibrium between the. Collect experimental data and create a solubility curve. Compare the number of ions in solution. for this experiment, your students will explore basic chemistry concepts by testing the solubility of different substances in water. solubility tests can suggest the size and polarity of an unknown compound and the presence of basic or. in this experiment, the relative solubility (and an approximate value of the ksp) of lead iodide will be determined by direct. hot or cold, which water is better for soluble substances? add different salts to water, then watch them dissolve and achieve a dynamic equilibrium with solid precipitate. Explore your finding from this practical into the effect of temperature on solubility.
Bile Solubility Test Principle, Procedure, Results Microbe Online
Solubility Test Lab solubility tests can suggest the size and polarity of an unknown compound and the presence of basic or. The key to solubility is that, when a saturated solution is present, it is essentially the point where you are at an equilibrium between the. hot or cold, which water is better for soluble substances? Collect experimental data and create a solubility curve. in this experiment, the relative solubility (and an approximate value of the ksp) of lead iodide will be determined by direct. Explore your finding from this practical into the effect of temperature on solubility. solubility tests can suggest the size and polarity of an unknown compound and the presence of basic or. add different salts to water, then watch them dissolve and achieve a dynamic equilibrium with solid precipitate. for this experiment, your students will explore basic chemistry concepts by testing the solubility of different substances in water. Compare the number of ions in solution.
From www.youtube.com
Solubility Lab Visuals YouTube Solubility Test Lab solubility tests can suggest the size and polarity of an unknown compound and the presence of basic or. The key to solubility is that, when a saturated solution is present, it is essentially the point where you are at an equilibrium between the. Compare the number of ions in solution. hot or cold, which water is better for. Solubility Test Lab.
From julietsobel.blogspot.com
Juliet's Chemistry Blog Constructing A Solubility Curve Lab Solubility Test Lab Explore your finding from this practical into the effect of temperature on solubility. Collect experimental data and create a solubility curve. add different salts to water, then watch them dissolve and achieve a dynamic equilibrium with solid precipitate. for this experiment, your students will explore basic chemistry concepts by testing the solubility of different substances in water. . Solubility Test Lab.
From www.youtube.com
Solubility Tests for Organic Compounds YouTube Solubility Test Lab in this experiment, the relative solubility (and an approximate value of the ksp) of lead iodide will be determined by direct. solubility tests can suggest the size and polarity of an unknown compound and the presence of basic or. add different salts to water, then watch them dissolve and achieve a dynamic equilibrium with solid precipitate. . Solubility Test Lab.
From www.testmould.com
Bitumen solubility test apparatus Tianpeng Solubility Test Lab The key to solubility is that, when a saturated solution is present, it is essentially the point where you are at an equilibrium between the. in this experiment, the relative solubility (and an approximate value of the ksp) of lead iodide will be determined by direct. for this experiment, your students will explore basic chemistry concepts by testing. Solubility Test Lab.
From www.youtube.com
Laboratory Exercise Testing the Solubility of the Substance Philippines YouTube Solubility Test Lab Compare the number of ions in solution. add different salts to water, then watch them dissolve and achieve a dynamic equilibrium with solid precipitate. for this experiment, your students will explore basic chemistry concepts by testing the solubility of different substances in water. Collect experimental data and create a solubility curve. Explore your finding from this practical into. Solubility Test Lab.
From www.youtube.com
Solubility Lab 2 part 1 test tube 2 YouTube Solubility Test Lab The key to solubility is that, when a saturated solution is present, it is essentially the point where you are at an equilibrium between the. solubility tests can suggest the size and polarity of an unknown compound and the presence of basic or. in this experiment, the relative solubility (and an approximate value of the ksp) of lead. Solubility Test Lab.
From ctdbfredeen.blogspot.com
Lab 20 Solubility Inquiry Lab Solubility Test Lab solubility tests can suggest the size and polarity of an unknown compound and the presence of basic or. Compare the number of ions in solution. in this experiment, the relative solubility (and an approximate value of the ksp) of lead iodide will be determined by direct. The key to solubility is that, when a saturated solution is present,. Solubility Test Lab.
From www.docsity.com
Solubility General Chemistry Lecture Slides Docsity Solubility Test Lab Collect experimental data and create a solubility curve. add different salts to water, then watch them dissolve and achieve a dynamic equilibrium with solid precipitate. in this experiment, the relative solubility (and an approximate value of the ksp) of lead iodide will be determined by direct. Explore your finding from this practical into the effect of temperature on. Solubility Test Lab.
From www.streck.com
SICKLEDEX Testing Kit for Hemoglobin S Streck Solubility Test Lab add different salts to water, then watch them dissolve and achieve a dynamic equilibrium with solid precipitate. The key to solubility is that, when a saturated solution is present, it is essentially the point where you are at an equilibrium between the. hot or cold, which water is better for soluble substances? for this experiment, your students. Solubility Test Lab.
From www.numerade.com
SOLVED Text EXPERIMENT 1 SOLUBILITY TESTING Data Sheet Table 1. Hydrocarbon Solubility Test Solubility Test Lab for this experiment, your students will explore basic chemistry concepts by testing the solubility of different substances in water. hot or cold, which water is better for soluble substances? solubility tests can suggest the size and polarity of an unknown compound and the presence of basic or. in this experiment, the relative solubility (and an approximate. Solubility Test Lab.
From studylib.net
Solubility Lab Solubility Test Lab solubility tests can suggest the size and polarity of an unknown compound and the presence of basic or. in this experiment, the relative solubility (and an approximate value of the ksp) of lead iodide will be determined by direct. hot or cold, which water is better for soluble substances? add different salts to water, then watch. Solubility Test Lab.
From www.coursehero.com
[Solved] . Report Reactions Of Hydrocarbons Solubility Tests From the... Course Hero Solubility Test Lab in this experiment, the relative solubility (and an approximate value of the ksp) of lead iodide will be determined by direct. Explore your finding from this practical into the effect of temperature on solubility. Collect experimental data and create a solubility curve. The key to solubility is that, when a saturated solution is present, it is essentially the point. Solubility Test Lab.
From www.studocu.com
Lab Report 1 Solubility Tam Huynh Partner Lindsey Campbell Prof. Dutz CHEM February 11 Solubility Test Lab hot or cold, which water is better for soluble substances? solubility tests can suggest the size and polarity of an unknown compound and the presence of basic or. Compare the number of ions in solution. in this experiment, the relative solubility (and an approximate value of the ksp) of lead iodide will be determined by direct. . Solubility Test Lab.
From shanghaichangji.en.made-in-china.com
Lab Tests Apparatus of solubility on Bitumen for Pavement Construction, solubility test for Solubility Test Lab Compare the number of ions in solution. solubility tests can suggest the size and polarity of an unknown compound and the presence of basic or. The key to solubility is that, when a saturated solution is present, it is essentially the point where you are at an equilibrium between the. in this experiment, the relative solubility (and an. Solubility Test Lab.
From studylib.net
Solubility Lab Solubility Test Lab for this experiment, your students will explore basic chemistry concepts by testing the solubility of different substances in water. solubility tests can suggest the size and polarity of an unknown compound and the presence of basic or. Collect experimental data and create a solubility curve. in this experiment, the relative solubility (and an approximate value of the. Solubility Test Lab.
From www.youtube.com
Solubility test YouTube Solubility Test Lab for this experiment, your students will explore basic chemistry concepts by testing the solubility of different substances in water. Compare the number of ions in solution. The key to solubility is that, when a saturated solution is present, it is essentially the point where you are at an equilibrium between the. solubility tests can suggest the size and. Solubility Test Lab.
From www.youtube.com
SOLUBILITY TEST YouTube Solubility Test Lab hot or cold, which water is better for soluble substances? solubility tests can suggest the size and polarity of an unknown compound and the presence of basic or. add different salts to water, then watch them dissolve and achieve a dynamic equilibrium with solid precipitate. Compare the number of ions in solution. Collect experimental data and create. Solubility Test Lab.
From www.researchgate.net
The apparatus used to determine solubility. Download Scientific Diagram Solubility Test Lab Compare the number of ions in solution. Explore your finding from this practical into the effect of temperature on solubility. in this experiment, the relative solubility (and an approximate value of the ksp) of lead iodide will be determined by direct. hot or cold, which water is better for soluble substances? add different salts to water, then. Solubility Test Lab.